What Is AR 3125?
The Accreditation Requirements for Forensic Testing and Calibration, AR 3125, supplements ISO/IEC 17025:2017 with applicable requirements from the International Laboratory Accreditation Cooperation (ILAC) policies. Additionally,…

The Accreditation Requirements for Forensic Testing and Calibration, AR 3125, supplements ISO/IEC 17025:2017 with applicable requirements from the International Laboratory Accreditation Cooperation (ILAC) policies. Additionally,…

Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. As part of process validation through IQ/OQ/PQ,…

With the rise of “informatics” disciplines such as bioinformatics, cheminformatics, and health informatics, laboratory informatics is a critical part of today’s laboratory operations, helping ensure high quality and reliable…

Why Do You Need a Management Review? Would you fly or sail over the oceans without instruments to navigate your way? A small error at…

ISO/IEC 17025:2017 requires laboratories to calibrate measuring equipment when the measurement accuracy or uncertainty affects the validity of results and/or the metrological traceability of results…
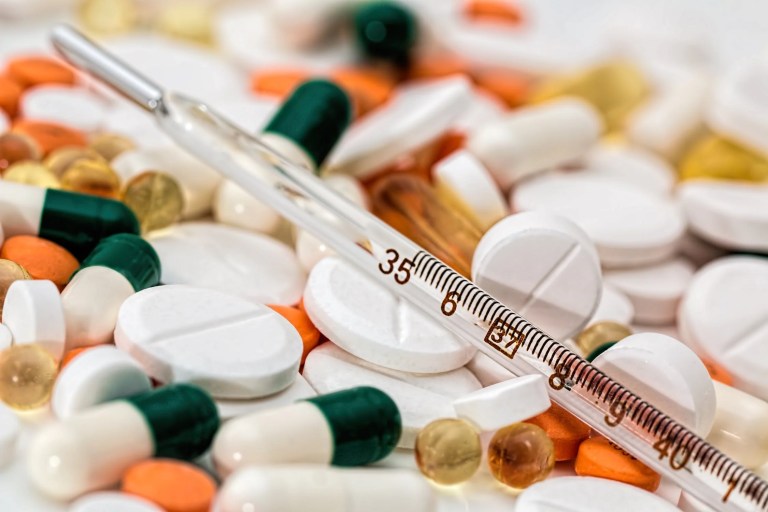
Concerns related to the efficacy and safety of medical drugs have caused most governments to develop regulatory agencies to oversee development and marketing of drug products and…

Reagent bottles are most commonly used to store chemical reagents, including acid and alkali solvents that can be safely stored due to anti-corrosion capabilities. These bottles are…

Any changes to a fixed scope of accreditation would require the laboratory to contact their accreditation body requesting a scope expansion to add changes to…

Until 2019, the kelvin (K) was defined as “the fraction 1/273.16 of the thermodynamic temperature of the triple point of water.” The temperature measurement world…