ASTM D6954-24: Plastic Biodegradability
Using oxo-biodegradable technology can prevent future contributions to the accumulation of plastic waste that has escaped into the environment. Oxo-biodegradable plastic serves as a solution…

Using oxo-biodegradable technology can prevent future contributions to the accumulation of plastic waste that has escaped into the environment. Oxo-biodegradable plastic serves as a solution…

ISO/IEC 17011 is an international standard that specifies the requirements for competence, consistent operation, and impartiality of accreditation bodies assessing and accrediting conformity assessment bodies….

ANAB offers private training to clients in management systems, laboratories, credentialing, inspection bodies, and more. Organizations seek out private training for many reasons: Which ANAB…

Internal audits are required by many ISO certification and accreditation standards, such as ISO 9001 and ISO/IEC 17025. This stresses the importance of a vast…
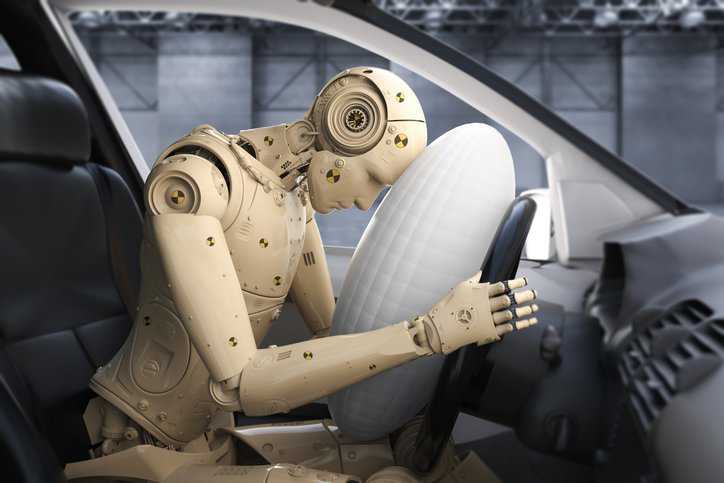
Each year, The National Highway Traffic Safety Administration (NHTSA) tests roughly 90 to 150 cars. It evaluates each vehicle is evaluated in four areas—frontal crash test, side barrier…

The DNA Identification Act of 1994 required the FBI laboratory to ensure that all DNA laboratories who receive federal grant funding or who participate in…

Anticoagulants are medicines that help prevent blood clots. They are given to people at high risk of blood clots, reducing their chances of developing serious,…

What Is a Remote Assessment? ISO/IEC 17011:2017 defines remote assessment as an assessment of the physical location or virtual site of a conformity assessment body…

The Accreditation Requirements for Forensic Testing and Calibration, AR 3125, supplements ISO/IEC 17025:2017 with applicable requirements from the International Laboratory Accreditation Cooperation (ILAC) policies. Additionally,…