IEC 60601-2-57 Ed. 2.0 b:2023— Medical Electrical Equipment
Patients in hospitals can be exposed to electromagnetic fields and optical radiation. This exposure may be associated with risks and hazards. IEC 60601-2-57 Ed. 2.0…
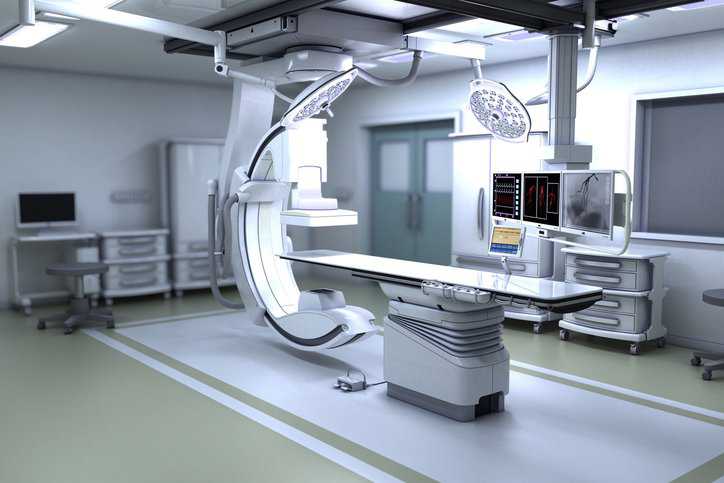
Patients in hospitals can be exposed to electromagnetic fields and optical radiation. This exposure may be associated with risks and hazards. IEC 60601-2-57 Ed. 2.0…
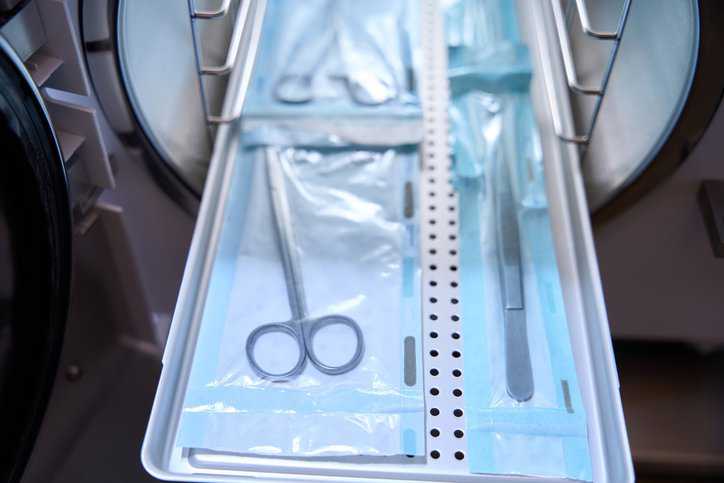
Ethylene oxide (EO or EtO) gas is one of the most common ways to sterilize medical devices, as approximately 50% of sterile medical devices are treated…
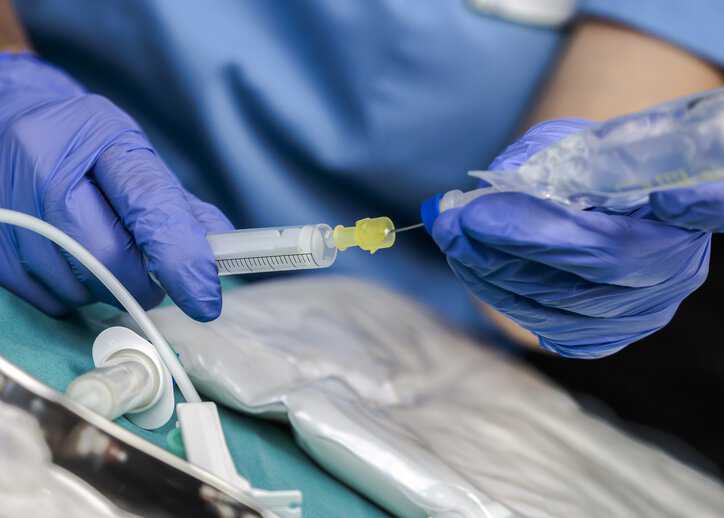
Intravascular catheters are an indispensable tool in modern-day medical practice, both for patients with acute injuries and for those with chronic illnesses. Approximately 90% of patients…

Medical device manufacturers are required to design and build quality into their products. This means that they must have processes and procedures in place to ensure…
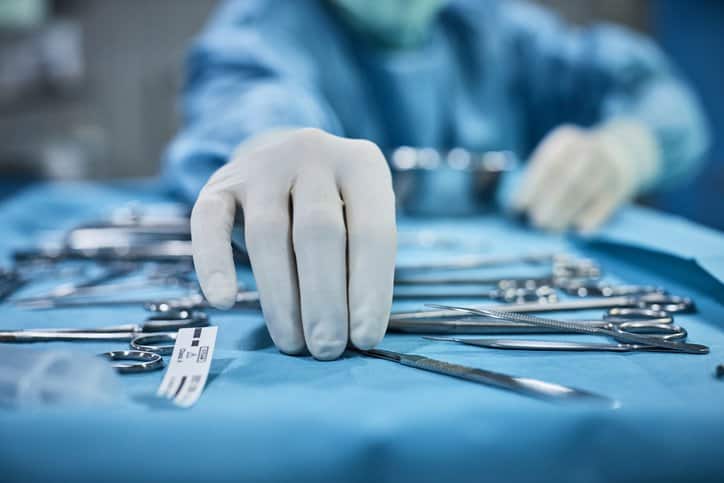
Flint knives, saws, and drills were used for surgery, amputation, and trepanation during the Upper Paleolithic and Mesolithic times in 6000 BCE. These were the…
The steps that involve manufacturing, regulation, planning, assessment, acquisition, and management of medical devices are complex but essential to guarantee their quality, safety, and compatibility…

Some 16 million people work in the healthcare sector, doing the work that keeps the rest of us alive. Personal protective equipment (PPE), such as…
Here are some facts about missing teeth you should know: the average American has lost 12 teeth by age 50; 69% of American adults are…

You are taller in the morning and in outer space. The lack of gravity from lying down to sleep or being in outer space causes…