ISO 15223-1:2021—Medical Devices Symbols
Symbols on medical devices are crucial for patient safety, regulatory compliance, and effective communication, allowing for clear and concise information across different languages and cultures. ISO…
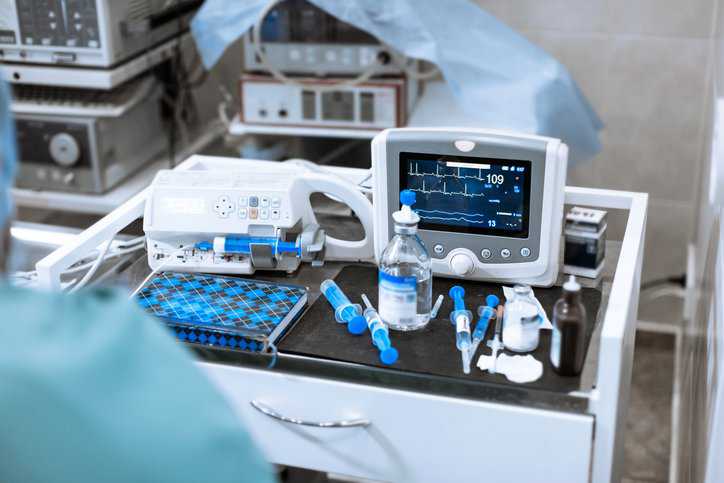
Symbols on medical devices are crucial for patient safety, regulatory compliance, and effective communication, allowing for clear and concise information across different languages and cultures. ISO…

Electrical safety is paramount in healthcare facilities because faulty electrical equipment can directly harm patients and staff through electric shock, burns, or even death. AAMI ESM4…
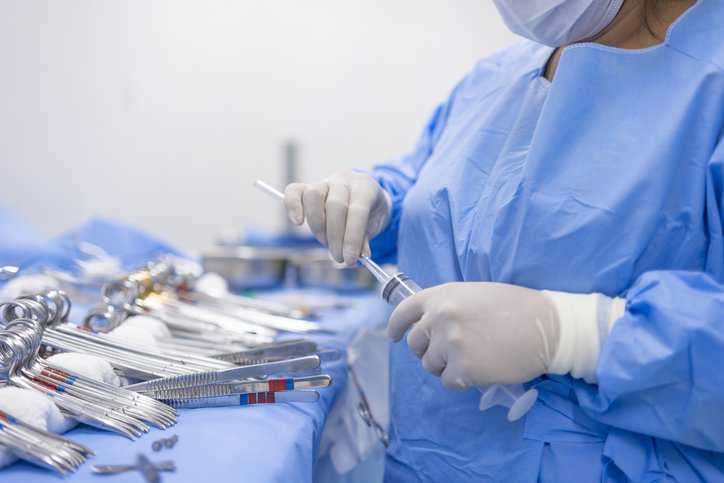
Achieving disinfection and sterilization through the use of disinfectants and sterilization practices is critical for preventing medical and surgical instruments from transmitting infectious pathogens to…

Healthcare facilities decontaminate and sterilize reusable medical devices numerous times prior to use. The reprocessing of medical devices includes several processes, and water quality plays…
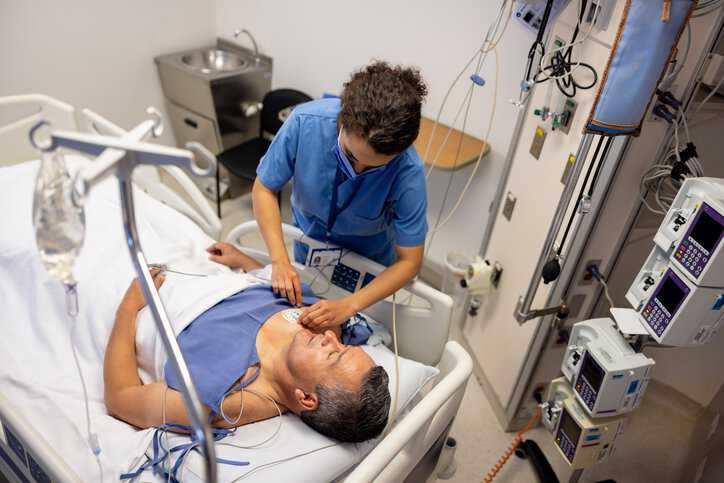
Chronic pain can be a debilitating condition that affects millions of people worldwide. Fortunately, advancements in neurostimulation technologies are providing relief to an unprecedented number of…
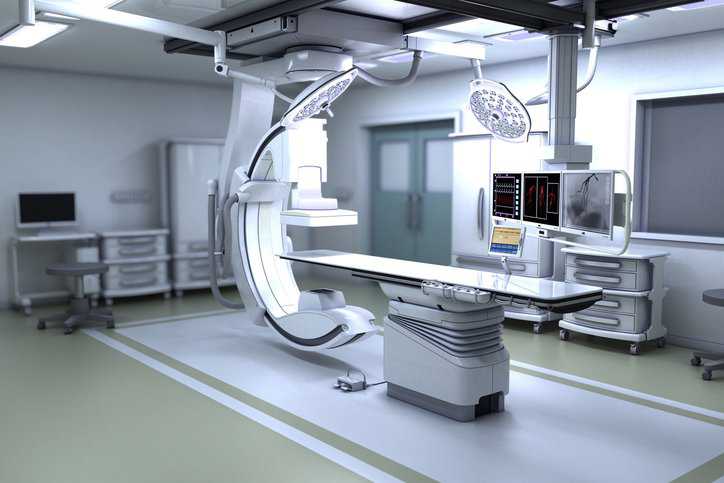
Patients in hospitals can be exposed to electromagnetic fields and optical radiation. This exposure may be associated with risks and hazards. IEC 60601-2-57 Ed. 2.0…
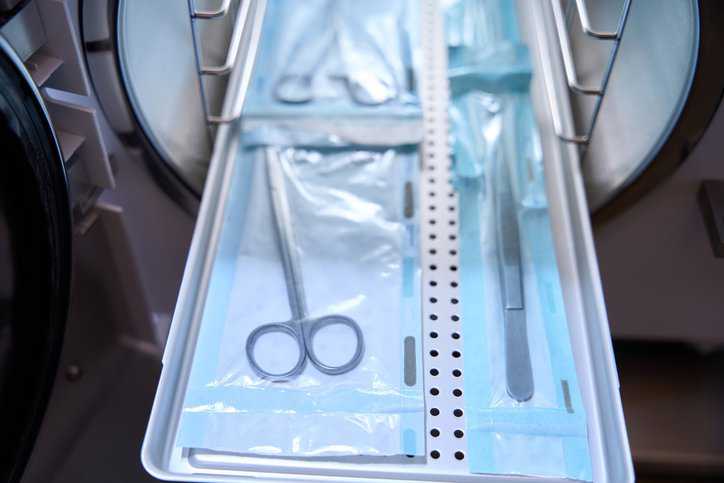
Ethylene oxide (EO or EtO) gas is one of the most common ways to sterilize medical devices, as approximately 50% of sterile medical devices are treated…
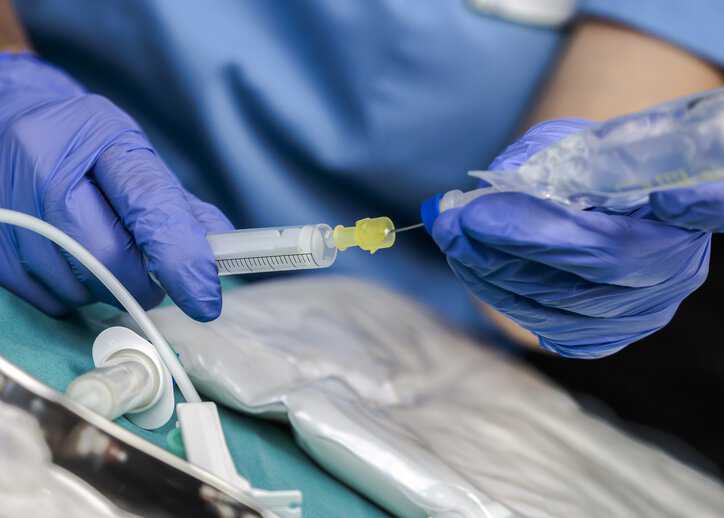
Intravascular catheters are an indispensable tool in modern-day medical practice, both for patients with acute injuries and for those with chronic illnesses. Approximately 90% of patients…

Medical device manufacturers are required to design and build quality into their products. This means that they must have processes and procedures in place to ensure…