ISO 10993-1:2025—Biological Evaluation of Medical Devices
Medical devices range from simple bandages to complex implantable devices like pacemakers and prosthetics. Regardless of their complexity, all medical devices must undergo rigorous testing…

Medical devices range from simple bandages to complex implantable devices like pacemakers and prosthetics. Regardless of their complexity, all medical devices must undergo rigorous testing…

Medical devices are essential to modern healthcare, but their safety and effectiveness rely heavily on human factors engineering (HFE). HFE helps reduce use errors (a…

3D printing technology has caused a revolution in health care delivery. It is being widely adopted for various applications, including anatomical modeling, surgical planning, the creation…
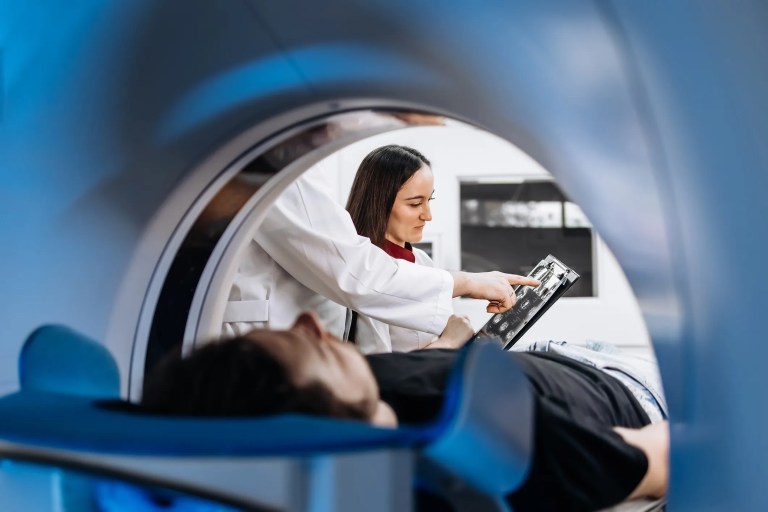
MRI’s history traces back to the 1930s with Isidor Rabi’s discovery of nuclear magnetic resonance, which demonstrated that atoms could emit radio waves in a magnetic…
Symbols on medical devices are crucial for patient safety, regulatory compliance, and effective communication, allowing for clear and concise information across different languages and cultures. ISO…

Electrical safety is paramount in healthcare facilities because faulty electrical equipment can directly harm patients and staff through electric shock, burns, or even death. AAMI ESM4…
Achieving disinfection and sterilization through the use of disinfectants and sterilization practices is critical for preventing medical and surgical instruments from transmitting infectious pathogens to…

Healthcare facilities decontaminate and sterilize reusable medical devices numerous times prior to use. The reprocessing of medical devices includes several processes, and water quality plays…
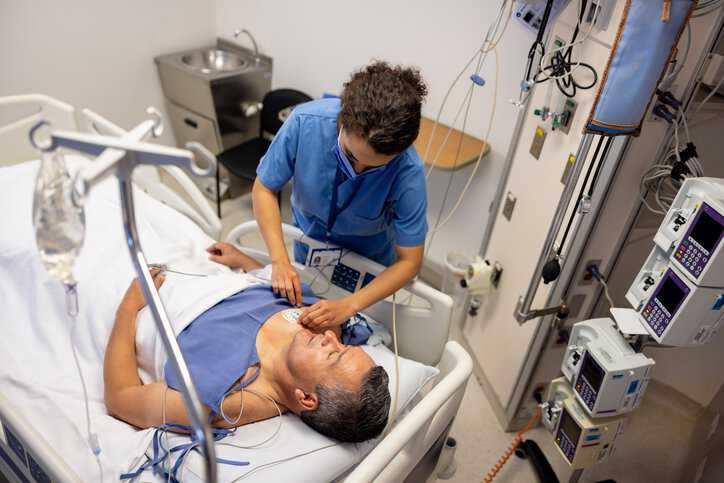
Chronic pain can be a debilitating condition that affects millions of people worldwide. Fortunately, advancements in neurostimulation technologies are providing relief to an unprecedented number of…