ISO 10993-1:2025—Biological Evaluation of Medical Devices
Medical devices range from simple bandages to complex implantable devices like pacemakers and prosthetics. Regardless of their complexity, all medical devices must undergo rigorous testing…

Medical devices range from simple bandages to complex implantable devices like pacemakers and prosthetics. Regardless of their complexity, all medical devices must undergo rigorous testing…

Medical devices are essential to modern healthcare, but their safety and effectiveness rely heavily on human factors engineering (HFE). HFE helps reduce use errors (a…

When disasters strike, emergency medical services (EMS) clinicians are among the first to respond. Whether stabilizing a patient at the scene of an accident or…

What Are Pharmaceutical Excipients in NSF/IPEC/ANSI 363? Pharmaceutical excipients are essential to the safety, quality, and efficacy of drug products, as they impact a variety…
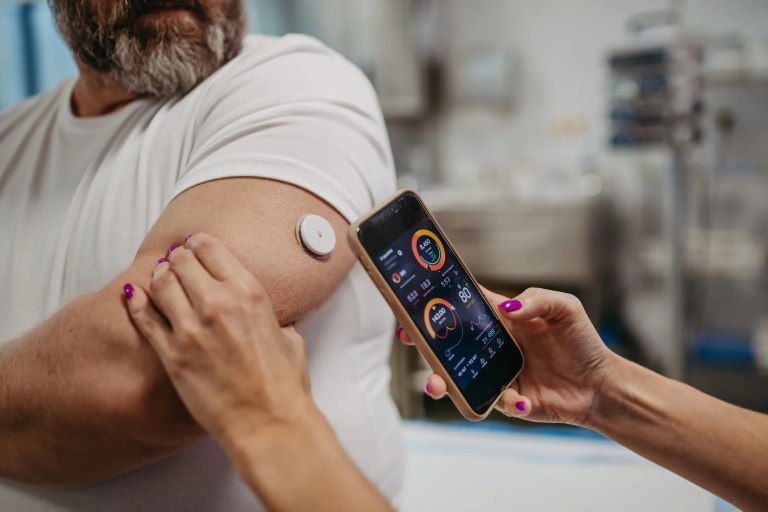
A growing number of people with diabetes are turning to wireless diabetes devices to monitor and manage their condition in an automated fashion. As diabetes…

Insulin pumps can be recommended for people with diabetes who have trouble managing their blood sugar levels or who have a lifestyle that makes it difficult…

In vitro means “in glass.” As such, in vitro diagnostics are tests usually conducted in test tubes and similar instruments. In vitro diagnostics (IVD) can…

38.4 million people have been diagnosed with diabetes in the United States. The number of people diagnosed with diabetes in the U.S. has increased in recent…
Patients in hospitals can be exposed to electromagnetic fields and optical radiation. This exposure may be associated with risks and hazards. IEC 60601-2-57 Ed. 2.0…