AAMI TIR17:2024—Sterilization of Health Care Products
Medical professionals are legally obligated to provide patients with a standard of care, which includes proper sterilization of medical equipment. This helps to assure that…
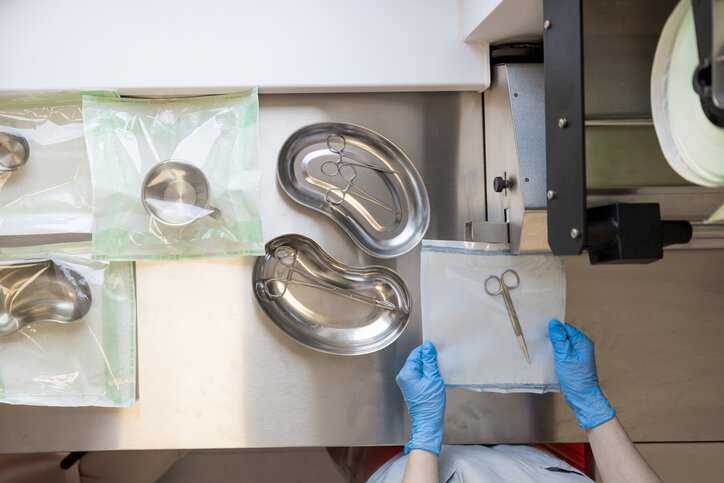
Medical professionals are legally obligated to provide patients with a standard of care, which includes proper sterilization of medical equipment. This helps to assure that…
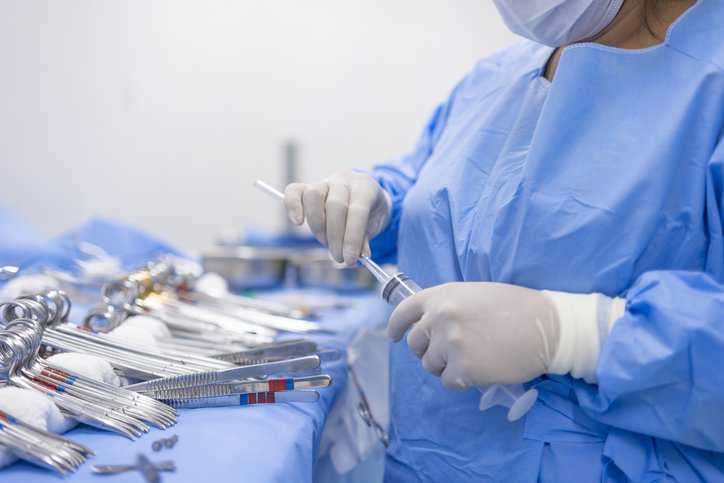
Achieving disinfection and sterilization through the use of disinfectants and sterilization practices is critical for preventing medical and surgical instruments from transmitting infectious pathogens to…
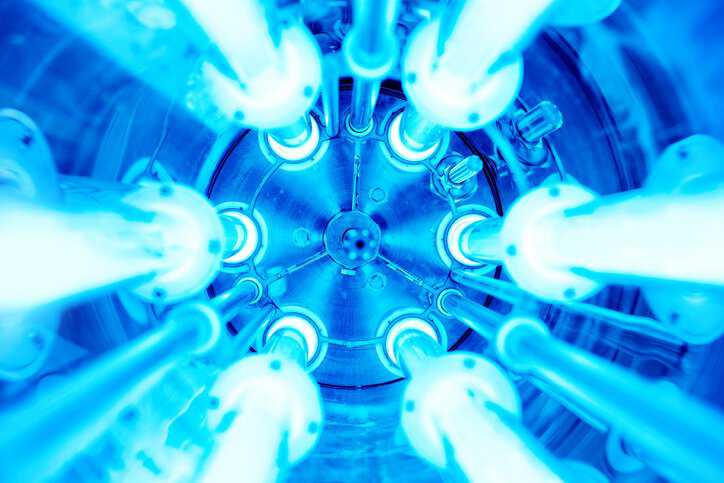
A radiation sterilization validation is used to determine the appropriate sterilization dose for a product, helping establish the pertinent sterilization parameters for health care reprocessing instructions….
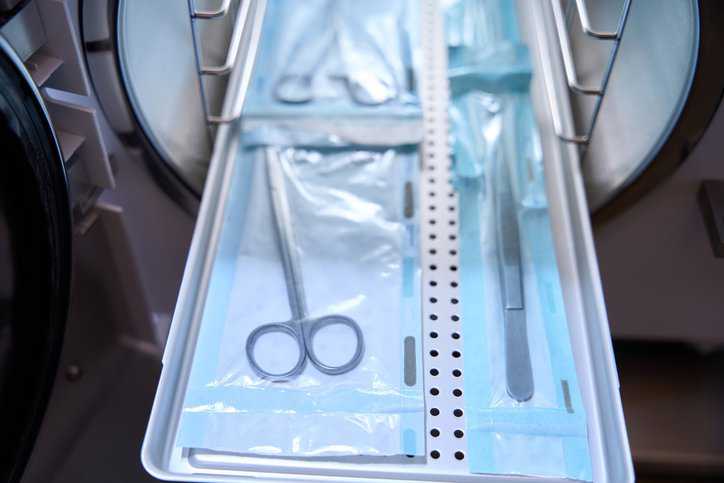
Ethylene oxide (EO or EtO) gas is one of the most common ways to sterilize medical devices, as approximately 50% of sterile medical devices are treated…

Both standards in the international series for the packaging for terminally sterilized medical devices have been revised: ISO 11607-1:2019, which addresses materials, sterile barrier systems,…
ANSI/AAMI/ISO 11737-1:2018 – Sterilization of health care products – Microbiological methods – Part 1: Determination of a population of microrganisms on products has been released….

A health care product is not sterile unless it is free of microorganisms. Sterilization is assured with these products and their materials and components through…
Human factors, or ergonomics, strives to understand the interactions among humans and the other elements of a system. Integrating human factors engineering, otherwise known as…
Guidance for Manufacturers For medical devices, sterilization is, unsurprisingly, crucial to patient health. For the best results, sterilization needs to begin with the manufacturers, who…