AAMI 80001-GS:2011— Getting Started With IEC 80001
Medical errors occur far too frequently: over 7 million American patients are impacted by medical errors each year; there are 7,000 to 9,000 Americans who…
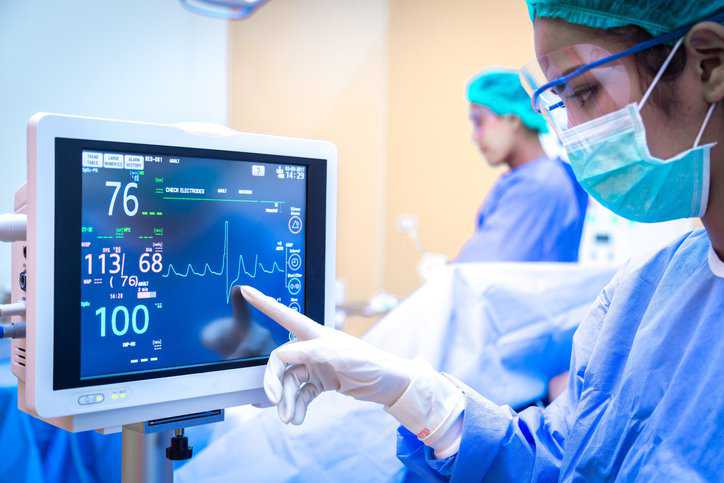
Medical errors occur far too frequently: over 7 million American patients are impacted by medical errors each year; there are 7,000 to 9,000 Americans who…

Hydrocolloids were the first elastic impression materials available to dentists. When mixed, they form a viscous liquid that can be seated over oral structures. The liquid…
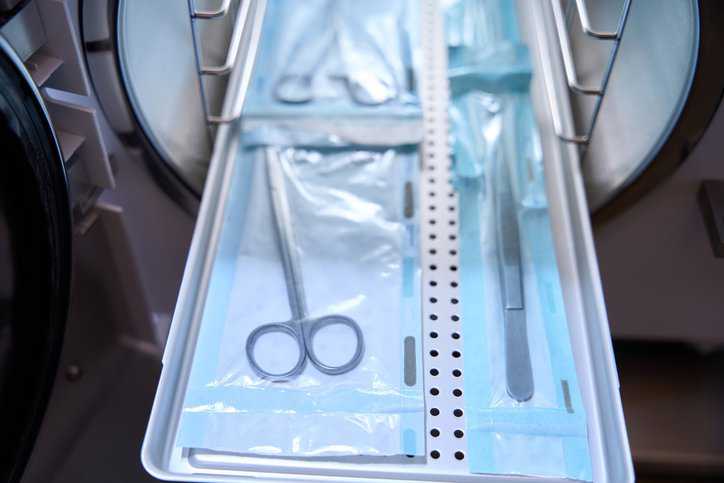
Ethylene oxide (EO or EtO) gas is one of the most common ways to sterilize medical devices, as approximately 50% of sterile medical devices are treated…

Rosa Damascena is the most widely used rose variety in perfumery. Classified in the rose family, Rosa Damascena rose essential oil is one of the best-known…
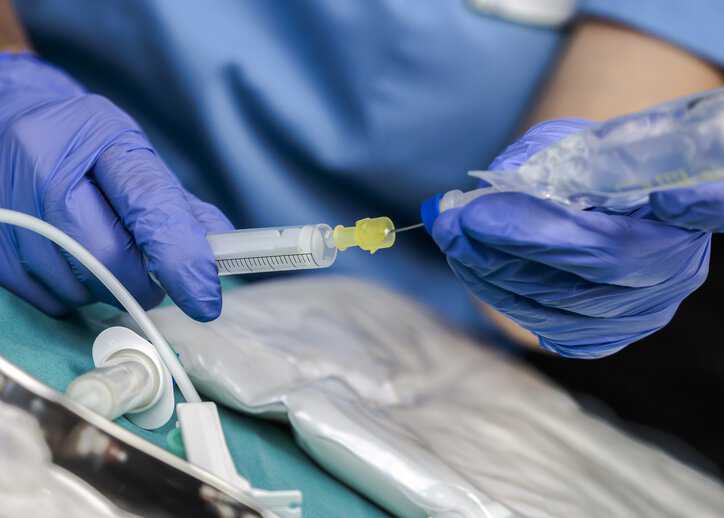
Intravascular catheters are an indispensable tool in modern-day medical practice, both for patients with acute injuries and for those with chronic illnesses. Approximately 90% of patients…
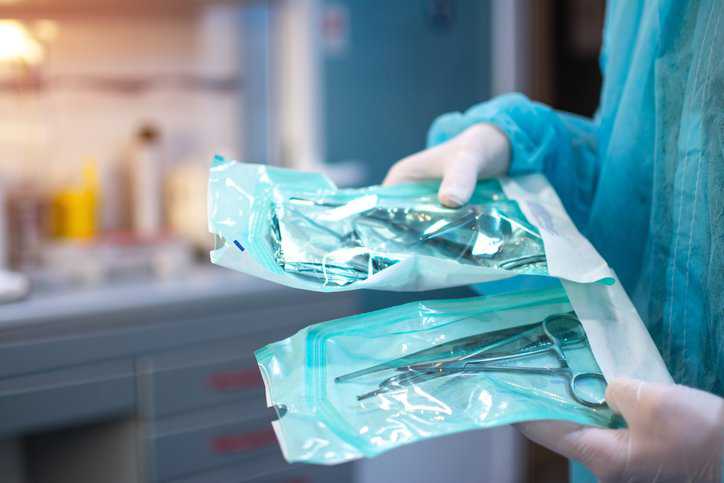
Products in pharmaceutical industries are prone to spoilage, and leaks in the packages or bottle seals can lead to air penetration, making the product unsafe for…

Medical device manufacturers are required to design and build quality into their products. This means that they must have processes and procedures in place to ensure…
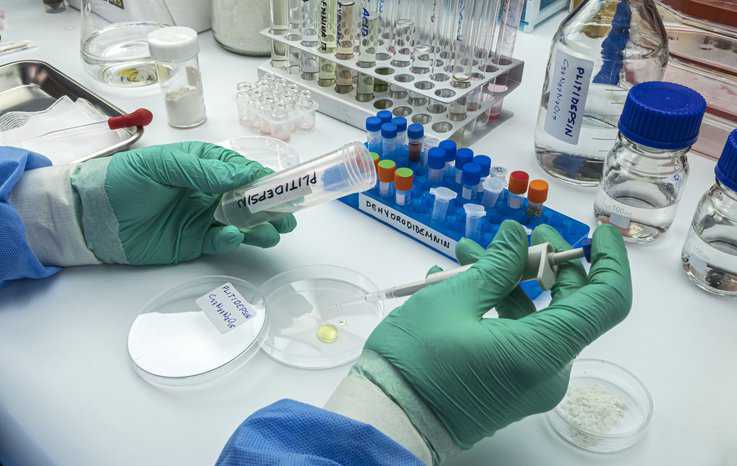
Risk assessment is a multi-step process which produces estimates of risk of an adverse health effect occurring as a consequence of ingesting, inhaling, and/or absorbing…
American National Standards developed by the American Dental Association allow patients across the nation to receive standardized care of the highest quality. ANSI/ADA 35-2019, for…