Symbols for Medical Device Labels
A voluntary consensus standard can result in the summation of the best existing practices and techniques in an industry that were in use prior to…

A voluntary consensus standard can result in the summation of the best existing practices and techniques in an industry that were in use prior to…
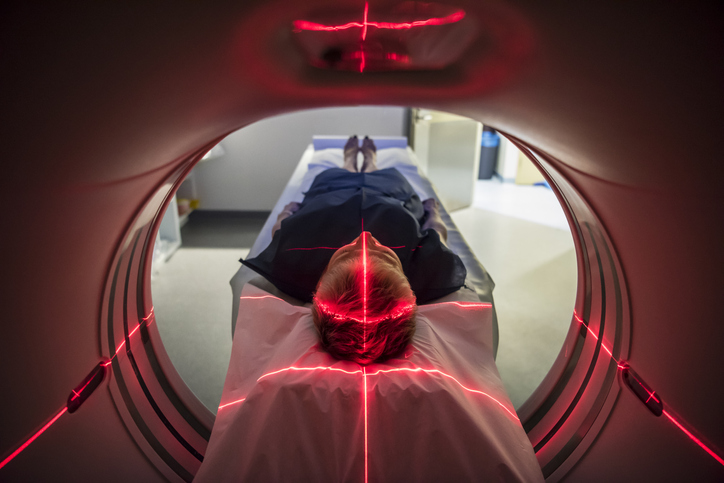
The medical industry follows a pattern of continuous advancement to meet patient needs, with innovations in medical technology plentiful at almost any point in time….

A consolidation of several previous standards, ANSI/AAMI ST79:2017 offers a Comprehensive Guide To Steam Sterilization And Sterility Assurance In Health Care Facilities. How Steam Sterilization…
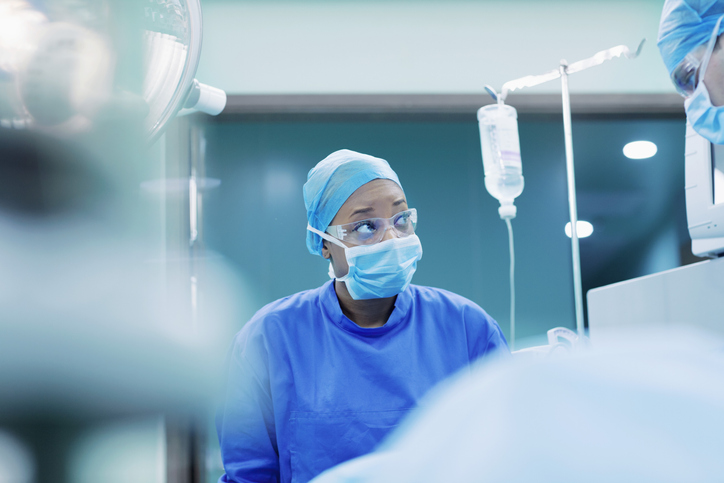
Patient care is a hands-on process, but this contact places unique stresses on healthcare professionals. Infection control practices, such as protective apparel, help protect patients…

Burgeoning alongside advancements in the medical industry itself, innovations in medical devices have blossomed over the past few centuries. Even before a rolled up notebook…
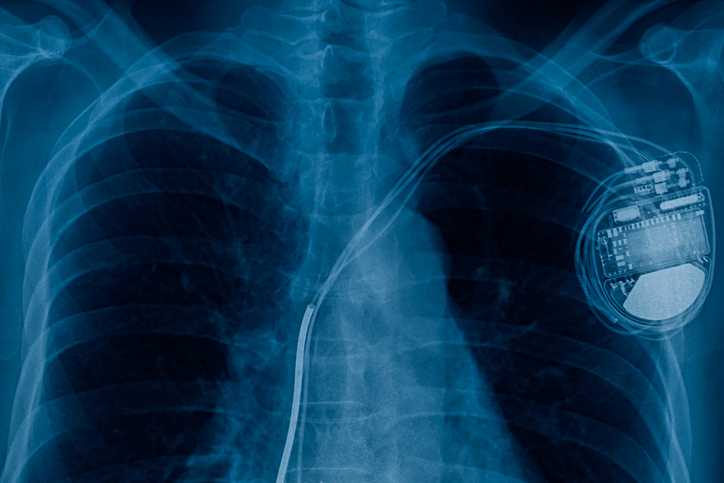
An international standard, ISO 14117:2019, outlines electromagnetic compatibility (EMC) test protocols for various active implantable medical devices. The heart typically pumps at 60-100 beats per…
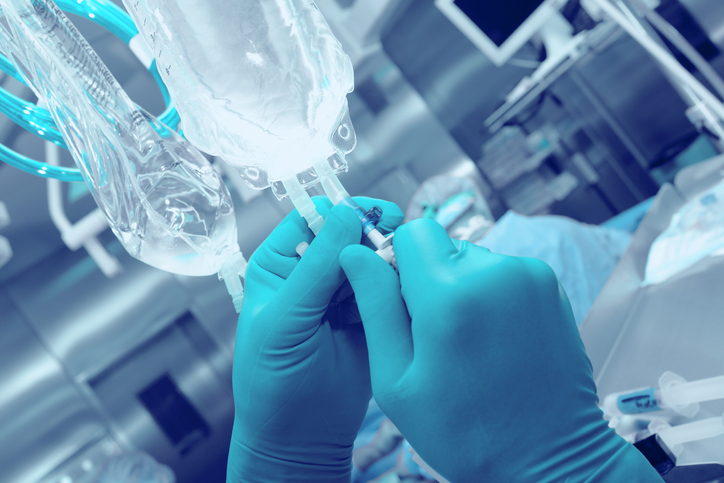
The standard for plastic containers for parenterals, ISO 15747:2018 – Plastic Containers For Intravenous Injections, has been revised. Intravenous (IV) medications are often optimal due…

The massive cloud migration of recent years has spread across a myriad of industries, with healthcare being one of the latest to adopt its systems…

Don’t let the name fool you. Test dummies, as anthropomorphic testing devices, are designed to suffer blunt force trauma, but they do so to acquire…